Author: Robby Berman / Source: Big Think

- A lot of Earth’s water is asteroidal in origin, but some of it may come from dissolved solar nebula gas.
- Our planet hides majority of its water inside: two oceans in the mantle and 4–5 in the core.
- New reason to suspect that water is abundant throughout the universe.
Scientists have puzzled for some time over how the Earth first acquired water. Some have theorized it arrived in cometary ice, or possibly aboard asteroids crashing onto the planet’s surface.
“But there’s another way to think about sources of water in the solar system’s formative days,” says Arizona State University’s Steven Desch, a member of the team of geoscientists led by Peter Buseck, Regents’ Professor at ASU’s School of Earth and Space Exploration. “Because water is hydrogen plus oxygen, and oxygen is abundant, any source of hydrogen could have served as the origin of Earth’s water.”
In a paper published in Journal of Geophysical Research, the researchers suggest that the H in our early H20 may have come from the rocky center of the planet itself, left there during its formation.
Heavy hydrogen
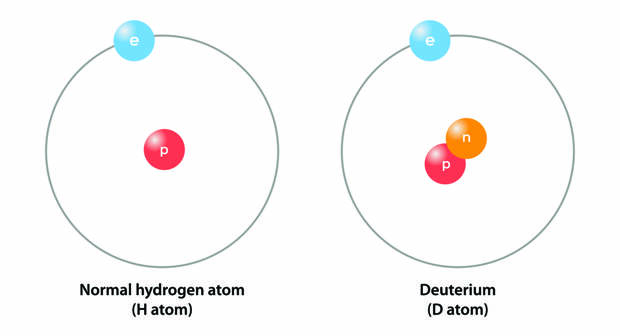
Image source: gritsalak karalak / Shutterstock
Lead author of the paper, Jun Wu, tells ASU, “The solar nebula has been given the least attention among existing theories, although it was the predominant reservoir of hydrogen in our early solar system.”
Earth has three major areas of water, the most visible of which is the ocean. There are two more “oceans,” however, under the ground dissolved into the mantle.
While both hold the liquid, the water above and below ground is not quite the same. It has to do with the presence of heavy hydrogen.While far and away most hydrogen atoms have a nucleus containing a single proton, the nucleus of about 1 in 7,000 hydrogen atoms has a neutron as well. These isotopes — exceptions to the single-proton norm — are considered “heavy” hydrogen atoms, or deuterium, abbreviated as “D.”
Scientists can ascertain the source of hydrogen by determining its ratio of D to A atoms, or its D/H ratio. The hydrogen in water from comets has a D/H ratio…
The post 1 in 100 water molecules started in solar nebulae appeared first on FeedBox.