Source: Good News Network
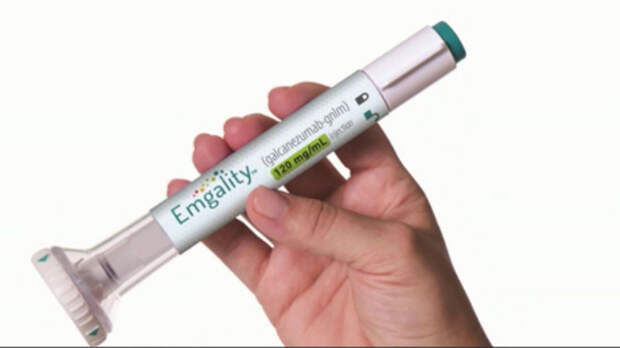
The U.S. Food and Drug Administration has approved a new preventative treatment for migraines in adults.
The treatment, which is called Emgality, is a once-monthly, self-administered injection that was developed by global pharmaceutical company Eli Lilly and Company.
“Despite the devastating impact of migraine, only about 10 percent of people living with the disease are currently taking a preventive treatment,” said Christi Shaw, president of Lilly Bio-Medicines.
“With this approval, we are thrilled to offer a preventive treatment option to adults living with this disease.”Migraine is a disabling, neurologic disease that affects more than 30 million American adults. According to the Medical…
The post FDA Approves Once-Monthly Preventative Migraine Treatment With No Major Side Effects appeared first on FeedBox.