Author: Laura Sanders / Source: Science News

A few months back, a new storefront appeared in my small Oregon town. Its shelves were packed with tinctures, jars of salve, coffee beans, bath bombs — even beard oil.
This motley collection shared a single star ingredient: CBD.Produced by the cannabis plant, CBD is the straitlaced cousin of marijuana’s more famous component — the THC that delivers a mind-swirling high. CBD, or cannabidiol, has no such intoxicating effects on the mind. Yet the molecule has captured people’s attention in a profound way, sold as a remedy for pain, anxiety, insomnia and other ailments — all without the high.
That neighborhood shop, CBD Scientific, is far from alone in its efforts to sell people on the benefits of CBD, which is found in both marijuana and hemp, two versions of the Cannabis sativa plant. CBD is popping up in products in pet stores, coffee shops and the health and beauty sections of mainstream grocery stores. It’s even being brewed into beer. I left the shop with a $5 bottle of water infused with “5,000,000 nanograms” of CBD.
So far, messages of CBD’s purported health benefits come from people trying to sell CBD products — not from scientists, says Margaret Haney, a neurobiologist who directs the Marijuana Research Laboratory at Columbia University. A gaping chasm separates the surging CBD market and the scientific evidence backing it. While there are reasons to be excited about CBD, the science just isn’t there yet, Haney says.
Booming business
U.S. sales of CBD-containing products are on the rise, and industry watchers expect a growing market in years to come. Epidiolex, an antiseizure drug made available in 2018, is the only prescription medication containing CBD. Sales figures beginning with 2018 are estimates.
U.S. CBD market growth and projections, 2014–2022
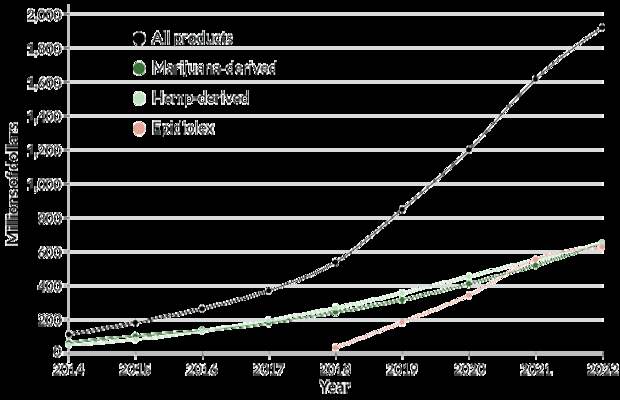
Source: New Frontier Data 2018, Hemp Business Journal
Scientists still don’t know all of the targets CBD hits in the human body, nor what effects it may have, if any. With the exception of tests in people with rare forms of epilepsy, large studies that compare CBD with placebos in people are rare. Much of the existing research was done with cells in the lab or in lab animals, with results that don’t necessarily translate to people.
And there’s always the chance that for some people, CBD’s magic is made not by the compound itself but by a powerful placebo effect; people who expect good outcomes are more likely to see benefits.
Researchers are stepping into the void, lured by promising early data. Small trials are under way looking at the effect of CBD on anxiety, pain, opioid addiction, depression and other health problems. National Institutes of Health funding for CBD studies went from zero in 2014 to an estimated $16 million in 2018.

“We’re very interested in CBD,” says Susan Weiss, director of the Division of Extramural Research at the National Institute on Drug Abuse in Bethesda, Md. Still, she urges caution to people eager to try CBD. Because of lax oversight, there’s no telling what’s inside many of those tinctures, oils, rubs and foods for sale online and in stores. “A lot of the products that people are taking may not be what they think,” she says.
Despite the risks and warnings, it seems safe to say that the collective fascination with CBD isn’t going to wear off anytime soon. “People think it’s great for everything,” says cognitive neuroscientist Kent Hutchison of the University of Colorado Boulder. That can’t possibly be true, he says. “But I do think it’s going to be great for some things. We just need to figure out what those things are.”
Mystery molecule
Each morning, Samantha Montanaro of Portland, Ore., drops a CBD tincture under her tongue. “I’m kind of testing out my own body with this,” she says. “I’m finding that it really helps with anxiety and stress.”
Montanaro isn’t alone; CBD testimonials are increasingly easy to find. In 2016, Montanaro, now 35, cofounded Tokeativity, a global cannabis community for women. Back then, “CBD wasn’t even a thing,” she says. But the first sparks of the CBD movement caught fire fast. “It’s been pretty crazy to watch how things have evolved,” she says. Some bullish analysts predict that the CBD market in the United States will balloon from hundreds of millions of dollars in 2018 to almost $20 billion by 2022.
Ziva Cooper directs UCLA’s Cannabis Research Initiative and fields a lot of questions about CBD. Her answers invariably disappoint. “When I tell [people] we don’t have very much evidence in people, they’re actually surprised,” she says. When it comes to CBD’s benefits, “there’s actually very little out there to hang our hats on.”
The one exception is for rare forms of childhood epilepsy. Neurologist Elizabeth Thiele of Massachusetts General Hospital in Boston had a young patient who was having over 100 seizures a day. After other treatments had failed, the boy’s parents began searching for a source of CBD oil, which they desperately wanted to try after learning about promising early results in animals. The family flew to England, so the boy could try the CBD formulation made by GW Pharmaceuticals. The child’s results, Thiele says, were remarkable. After a week of CBD, his daily seizures had fallen to single digits.

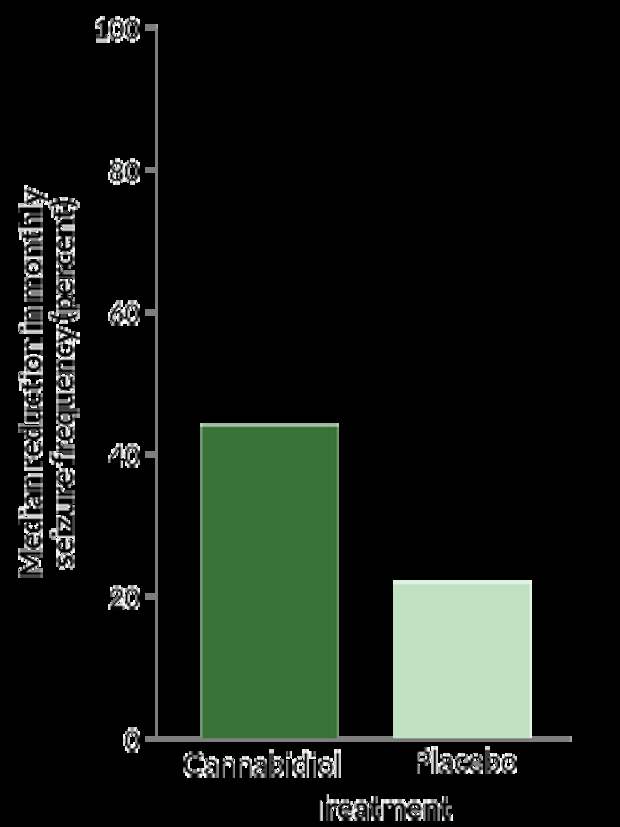
That result ultimately led to clinical trials, one of which included 171 people, mostly children, with Lennox-Gastaut syndrome, a rare and severe seizure disorder. In addition to their normal medication, half of the participants got doses of CBD that were rigorously tested and standardized by the drug’s maker. The other half received their regular treatment plus a placebo. After 14 weeks, the people taking CBD saw a median drop in monthly seizure frequency of about 44 percent; seizures in people who took the placebo dropped almost 22 percent. Thiele and her colleagues published those results in March 2018 in the Lancet.
Side effects were manageable, the researchers found. Diarrhea, sleepiness, poor appetite and vomiting were more likely to occur in the people who took CBD than in those who got the placebo. Along with results from several other trials, those data were strong enough to prompt the U.S. Food and Drug Administration to approve the CBD drug, called Epidiolex, last June.
In people with severe forms of epilepsy, CBD plus normal treatment (dark green bar) curbed seizures to a greater extent than did a placebo coupled with normal treatment (light green bar), one trial showed.
Reduction in seizure frequency during treatment
Despite rigorous testing of Epidiolex, big gaps in knowledge on how the…
The post The CBD boom is way ahead of the science appeared first on FeedBox.